Featured
Example Of An Allotrope
Example Of An Allotrope. Two types of carbon ring exist in it. In detail, it refers to different types of compounds made out of the same single element but in different chemical formulas and different arrangements.
For the element oxygen, there are two common allotropes as o2 and o3. Allotropes can be defined as different types of compounds made out of the same single element but in different chemical formulas and different arrangements. In all three allotropes, the carbon.
For Example, The Allotropes Of Carbon.
Allotropy (or allotropism) is when a chemical element can exist in two or more different forms in the same physical state or phase.these different forms are called allotropes.therefore, an allotrope is a different structure in which an element appears. The next higher temperature modification is called β, then γ and at still higher temperatures as δ (delta). An isotope is one way that protons and neutrons can form the same atomic element:
These Three Substances Right Here Are All Made Up Of Carbon, Just In Different Forms Or Allotropes.
Allotropes are different structural modifications of an element: ⋅ red phosphorus and white phosphorus are allotropes of phosphorus. Allotropes are two or more forms of the same element in the same physical state (solid, liquid, or gas) that differ from each other in their physical, and sometimes chemical, properties.
This Happens When The Atoms Of The Element Are Bonded Together In A Different Way.
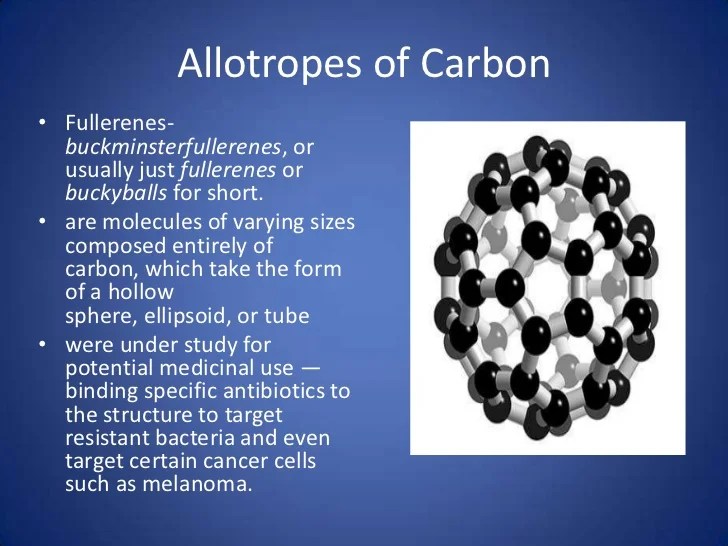
The most notable examples of allotropes are found in groups 14, 15, and 16 of the periodic table. In this video we’ll define the term allotrope, look at examples, and then practice so we can easil. This molecule contains 60 atoms of carbon.
A Synthetic Allotrope Of Carbon.
Allotropes are the different forms of a compound. Gaseous oxygen, for example, exists in three. Allotrope allotropes are two or more forms of the same element in the same physical state (solid, liquid, or gas) that differ from each other in their physical, and sometimes chemical, properties.
The Various Physical Forms Of Carbon Include Diamond And Graphite.
Allotropy, the existence of a chemical element in two or more forms, which may differ in the arrangement of atoms in crystalline solids or in the occurrence of molecules that contain different numbers of atoms. In this allotrope carbon atoms are arranged in a football shape. In essence, allotropy or allotropes pure elements that exist in two or more different physical forms.
Popular Posts
An Example Of A Two Point Violation Includes Reckless Driving
- Get link
- X
- Other Apps
Comments
Post a Comment