Featured
What Is An Example Of A Strong Base
What Is An Example Of A Strong Base. A monoacidic base is a base that produces one hydroxide ion when one of its molecules undergoes complete ionisation. H₃o⁺(aq) + oh⁻(aq) → 2h₂o(l).
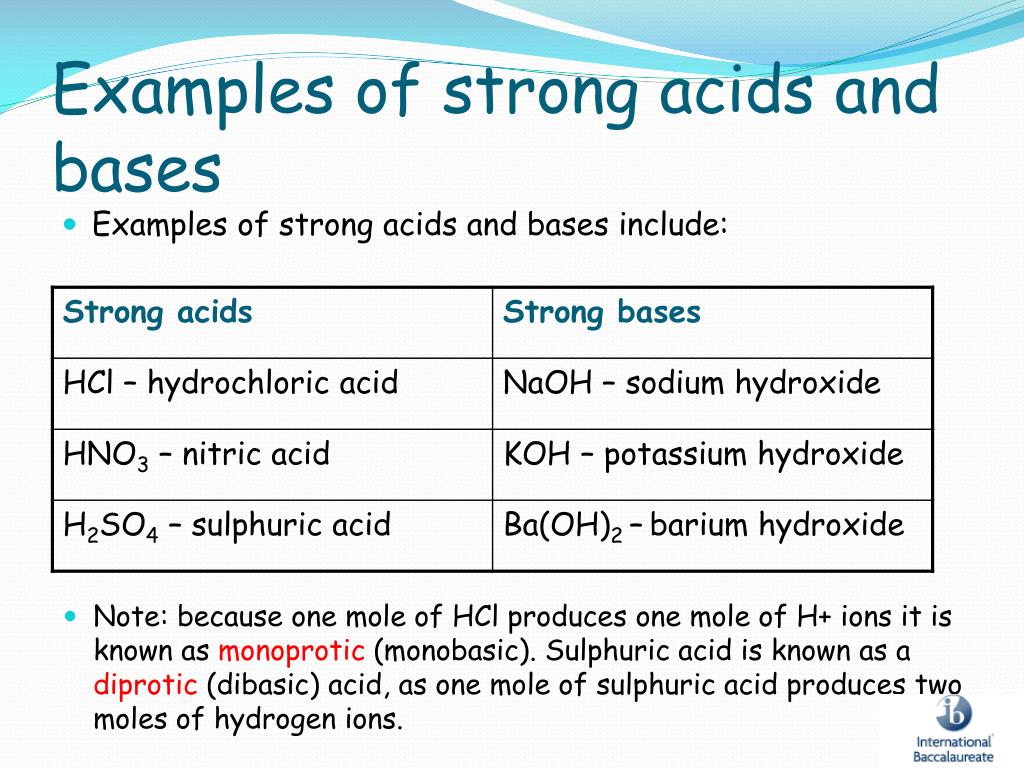
Strong bases are commonly, though not exclusively, formed from the hydroxides of alkali metals and alkaline earth metals. What are monoacidic, diacidic, and triacidic bases? The ionic compounds that produce negative hydroxide (oh −) ions when.
Salts Formed From Those Conjugate Bases Often Have A Ph Of 7, Or Just Above 7.
A monoacidic base is a base that produces one hydroxide ion when one of its molecules undergoes complete ionisation. The conjugate base of an acid, is the ion that results when the acid loses a proton. A weak acid or a weak base only partially dissociates.
Strong Bases Are Those Which Dissociate Fully When Dissolved In Water.
It’s only that it’s the oldest. They form hydroxide ions and increase the concentration of hydroxide in the water. The strong acids are perchloric acid, nitric acid, sulfuric acid, hydrochloric acid, hydroiodic acid and hydrobromic acid.
Some Examples Of Common Products That Contain Arrhenius Bases Include:
For example, if solid sodium. Examples of bases table of contents. A strong base is a base that is completely dissociated in an aqueous solution.
If Either The Acid Or The Base Is In Excess, The Ph Of The Resulting Solution Can Be Determined From The Concentration Of Excess Reactant.
In contrast, a weak base only partially dissociates into its ions in water. Strong bases react with strong acids to form stable. Superbases are stronger than hydroxide ions and cannot be kept in water;
In Aqueous Solution, Each Of These Essentially Ionizes 100%.
Ammonia is a good example of a weak base. At equilibrium, both the acid and the conjugate base are. Bases feel slippery and taste soapy.
Popular Posts
An Example Of A Two Point Violation Includes Reckless Driving
- Get link
- X
- Other Apps
Comments
Post a Comment